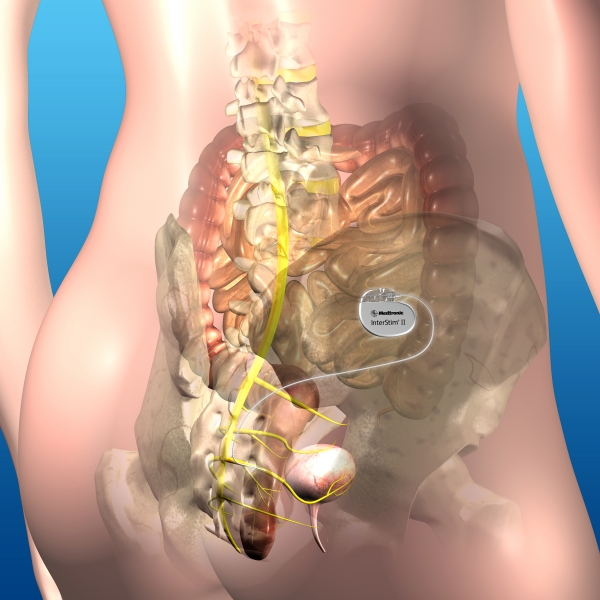
Sacral Nerve Stimulation

Sacral nerve stimulation (SNS) is a treatment alternative that may be indicated to improve bowel function. Impaired nerve function to the bowel may be a significant cause of faecal incontinence and sometimes constipation. Sacral nerve stimulation uses a small system, surgically implanted under the skin, to send mild electrical impulses to specific nerves via a special medical wire.
The sacral nerve stimulator is usually implanted under the skin in the upper buttock, where it is most comfortable and least visible. The device does not make any noise. It may be felt as a small bulge under your skin but it does not normally show through your clothes. Devices are 4.4 cm x 5.1 cm x 0.8 cm (height/ length/ thickness) and weigh 22 g.
The Sacral nerve stimulator comes with a remote control that allows you to adjust the stimulation produced by the system and turn the device on/off if necessary.
Trial (Evaluation phase)
The initial procedure is a minor operation to place a temporary medical wire in position – the end of the wire will be on the outside of your body secured with special surgical dressings. This will connect to a temporary stimulator device worn in a belt around your waist.
The procedure is done under a general anaesthetic as a day procedure, you should be comfortable to go home the same day and will be asked to keep a diary of your bowel function for the duration of the trial (usually 1 week).
You will be instructed how to use the patient remote control device to adjust the intensity of the stimulation and turn the SNS off if necessary.
You may be able to return to work whilst on the trial- but need to avoid physical exertion, bending, squatting, reaching and twisting which could result in movement of the temporary wire and impair the trial.
You wont be able to do a full shower or bath for the duration of the trial.
1 week later you will have a review appointment with Dr Meade to evaluate the trial and remove the temporary wire.
Permanent implant SNS device
After assessment of the trial SNS if you are proceeding to have the permanent device implanted this can be done within a couple of weeks or at a later time that is convenient.
This procedure is done under a general anaesthetic with discharge from hospital usually the following day.
The permanent SNS wire and device are fully implanted under the skin; simple waterproof dressings cover the incision sites and can be removed 5-7 days post operatively. You can shower but check that the dressings are fully intact to prevent water getting under the dressings.
You will have a patient remote control device to adjust settings and turn SNS off/on as necessary.
What can I expect after surgery?
Following your surgery, you will probably feel some minor wound pain/ discomfort at the incision site in your buttock, this should settle over a few days.
You may also feel some increased sensitivity at the stimulator implant site for two to six weeks after the surgery. This is caused by developing scar tissue and can occur with any type of implant surgery. It is your body's natural response to the implant, once the scar tissue forms; the discomfort will begin to disappear.
During the first six to eight weeks following the implantation, please avoid activities that may cause disruption to the SNS wire, i.e. heavy lifting, bending, squatting, reaching and twisting movements. This allows time for scar tissue to form and anchor the wire in place.
After the initial six to eight weeks you should continue to use some caution with these types of movements. Once your incision has healed, the stimulator site requires no special care. However, you should talk to your doctor if you perform any excessive or repetitive activities that may damage your stimulator or the medical wire.
What are the risks?
Implanting an InterStim (SNS) system has risks similar to any surgical procedure, including swelling, bruising, bleeding and infection, adverse events can include;
Please contact our rooms or seek medical attention after hours if you experience any of the following concerns;
A typical follow-up schedule is an initial 6-8 week post-op review and then six monthly to check and adjust the device.
Important considerations for Sacral Nerve Stimulation
SNS devices may be subject to interference from electromagnetic radiation (EMR) and radio frequency (RF EMR) emitted from other sources for example; power tools, screening devices in shops and airport.
The effect could be a short episode of uncomfortable stimulation from the SNS or the interference may cause the SNS to turn on or off.
This can be avoided by turning the SNS off and the amplitude to zero before the activity.
Many people who have a SNS report that there are minimal problems with interference and that they rarely need to alter the stimulator setting or turn the device off.
Some medical procedures are contraindicated for people with SNS including;
Magnetic resonance imaging (MRIs) – the radio frequency energy from the MRI may concentrate in the SNS medical wire and lead to heating and tissue damage. It may be possible to have a brain MRI but would require confirmation of the settings used for MRI and compatibility with the SNS.
Ultrasound should not be used over or near the SNS implant site, this may cause damage to the SNS device.
Electrocautery requires precautions to prevent damage to the SNS and tissue damage.
A full list of precautions is contained in the Patient therapy guide booklet, which is provided with the device. Always inform your other clinicians including dentists that you have an implanted neurostimulation system and take your therapy guide with you.
Always carry your patient control remote with you to be able to turn the SNS off/ on as needed.
SNS can be compatible with other surgical implant devices including cardiac pacemakers and defibrillators.
Sacral nerve stimulation is reversible i.e. the implant can be surgically removed if necessary.
Removal of the implanted device is billed separately at the time of any such surgery.
For more information, visit the Medtronic (Australia) website on about sacral neuromodulation (SNS) here.
RestoreYourFreedom is a helpful site where you can access patient ambassadors. You can ask questions about the SNS device by email and read their stories.